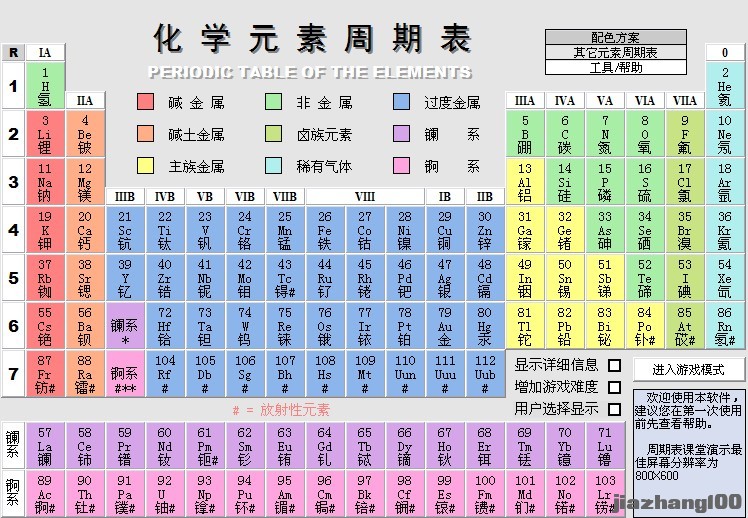
元素列表(来自百度百科)
| 原子序数 | 符号 | 中文 | 读音 | 相对原子质量 | 价电子 | 常见化合价 | 分类 | 英文名 | 简介 |
|---|
| 1 | H | 氢 | qīng | 1.008 | 1s1 | +1、-1 | 主族 非金属 | Hydrogen | 密度最小,同位素为氕、氘和氚 |
|---|
| 2 | He | 氦 | hài | 4.003 | 1s2 | 0 | 主族 非金属 稀有气体 | Helium | 最难液化,稀有气体,至今未发现化合物 |
|---|
3 | Li | 锂 | lǐ | 6.941 | 2s1 | +1 | 主族 金属 碱金属 | Lithium | 性质活泼 |
|---|
| 4 | Be | 铍 | pí | 9.012 | 2s2 | +2 | 主族 金属 碱土金属 | Beryllium | 最轻碱土金属元素 |
|---|
| 5 | B | 硼 | péng | 10.81 | 2s2 2p1 | +3 | 主族 非金属 | Boron | 单质硬度仅次于金刚石的非金属元素 |
|---|
| 6 | C | 碳 | tàn | 12.01 | 2s2 2p2 | 无机+2、+4、-4,有机不规则 | 主族 非金属 | Carbon | 硬度最高(金刚石),细胞干重中含量最高 |
|---|
| 7 | N | 氮 | dàn | 14.01 | 2s2 2p3 | -3、 +1 、+2、 +3、 +4、+5 | 主族 非金属 | Nitrogen | 空气中含量最多的元素 |
|---|
| 8 | O | 氧 | yǎng | 16.00 | 2s2 2p4 | -2、-1 | 主族 非金属 | Oxygen | 地壳中最多,生物体内最多 |
|---|
| 9 | F | 氟 | fú | 19.00 | 2s2 2p5 | -1 | 主族 非金属 卤素 | Fluorine | 最活泼的非金属,单质不能被氧化 |
|---|
| 10 | Ne | 氖 | nǎi | 20.18 | 2s2 2p6 | 0 | 主族 非金属 稀有气体 | Neon | 稀有气体 |
|---|
| 11 | Na | 钠 | nà | 22.99 | 3s1 | +1 | 主族 金属 碱金属 | Sodium | 活泼,与空气或水接触发生反应,只能储存在煤油或稀有气体中 |
|---|
| 12 | Mg | 镁 | měi | 24.31 | 3s2 | +2 | 主族 金属 碱土金属 | Magnesium | 轻金属之一 |
|---|
| 13 | Al | 铝 | lǚ | 26.98 | 3s2 3p1 | +3 | 主族 金属 | Aluminium | 地壳里含量最多的金属 |
|---|
| 14 | Si | 硅 | guī | 28.09 | 3s2 3p2 | +4、-4 | 主族 非金属 | Silicon | 地壳中含量仅次于氧 |
|---|
| 15 | P | 磷 | lín | 30.97 | 3s2 3p3 | -3、+3、+5 | 主族 非金属 | Phosphorus | 白磷有剧毒且在常温下可以自燃 |
|---|
| 16 | S | 硫 | liú | 32.06 | 3s2 3p4 | -2、+4、+6 | 主族 非金属 | Sulphur | 质地较软且轻。与氧气燃烧反应形成有毒的二氧化硫 |
|---|
| 17 | Cl | 氯 | lǜ | 35.45 | 3s2 3p5 | -1、+1、+3、+4、+5、+7 | 主族 非金属 卤素 | Chlorine | 有毒、活泼 |
|---|
| 18 | Ar | 氩 | yà | 39.95 | 3s2 3p6 | 0 | 主族 非金属 稀有气体 | Argon | 稀有气体,在空气中含量最多的稀有气体 |
|---|
| 19 | K | 钾 | jiǎ | 39.10 | 4s1 | +1 | 主族 金属 碱金属 | Potassium | 比钠活泼 |
|---|
| 20 | Ca | 钙 | gài | 40.08 | 4s2 | +2 | 主族 金属 碱土金属 | Calcium | 骨骼主要组成成分 |
|---|
| 21 | Sc | 钪 | kàng | 44.96 | 3d1 4s2 | +3 | 副族 金属 | Scandium | 一种柔软过渡金属,常与钆、铒混合存在 |
|---|
| 22 | Ti | 钛 | tài | 47.87 | 3d2 4s2 | +3、+4 | 副族 金属 | Titanium | 能在氮气中燃烧,熔点高 |
|---|
| 23 | V | 钒 | fán | 50.94 | 3d3 4s2 | +3、+5 | 副族 金属 | Vanadium | 高熔点稀有金属 |
|---|
| 24 | Cr | 铬 | gè | 52.00 | 3d5 4s1 | +3、+4、+6 | 副族 金属 | Chromium | 硬度最高的金属 |
|---|
| 25 | Mn | 锰 | měng | 54.94 | 3d5 4s2 | 区间[-3,+7]的整数 | 副族 金属 | Manganese | 在地壳中分布广泛 |
|---|
| 26 | Fe | 铁 | tiě | 55.85 | 3d6 4s2 | +2、+3、+6 | Ⅷ族 金属 | Iron | 地壳含量第二高的金属,单质产量最高,有磁性 |
|---|
| 27 | Co | 钴 | gǔ | 58.93 | 3d7 4s2 | +2、+3 | Ⅷ族 金属 | Cobalt | 同位素60Co被应用于X光发生器中,有磁性 |
|---|
| 28 | Ni | 镍 | niè | 58.69 | 3d8 4s2 | +2、+3 | Ⅷ族 金属 | Nickel | 有磁性和良好可塑性 |
|---|
| 29 | Cu | 铜 | tóng | 63.55 | 3d104s1 | +1、+2 | 副族 金属 | Copper | 人类发现较早的金属之一,可塑性很好 |
|---|
| 30 | Zn | 锌 | xīn | 65.39 | 3d10 4s2 | +2 | 副族 金属 | Zinc | 人体需要的微量元素 |
|---|
| 31 | Ga | 镓 | jiā | 69.72 | 3d104s2 4p1 | +3 | 主族 金属 | Gallium | 熔点低沸点高 |
|---|
| 32 | Ge | 锗 | zhě | 72.64 | 3d104s2 4p2 | +4 | 主族 金属 | Germanium | 是一种重要的半导体材料 |
|---|
| 33 | As | 砷 | shēn | 74.92 | 4s2 4p3 | -3、+3、+5 | 主族 非金属 | Arsenic | As2O3(即砒霜)剧毒 |
|---|
| 34 | Se | 硒 | xī | 78.96 | 4s2 4p4 | -2、+4、+6 | 主族 非金属 | Selenium | 可使玻璃致色为鲜红色 |
|---|
| 35 | Br | 溴 | xiù | 79.90 | 4s2 4p5 | -1、+5、+7 | 主族 非金属 卤素 | Bromine | 活泼,单质为红棕色液体 |
|---|
| 36 | Kr | 氪 | kè | 83.80 | 4s24p6 | +2 | 主族 非金属 稀有气体 | Krypton | 稀有气体 |
|---|
| 37 | Rb | 铷 | rú | 85.47 | 5s1 | +1 | 主族 金属 碱金属 | Rubidium | 比钾活泼 |
|---|
| 38 | Sr | 锶 | sī | 87.62 | 5s2 | +2 | 主族 金属 碱土金属 | Strontium | 是碱土元素中丰度最小的元素 |
|---|
| 39 | Y | 钇 | yǐ | 88.91 | 4d1 5s2 | +3 | 副族 金属 | Yttrium | 人工合成的钇铝榴石曾被当做钻石的替代品 |
|---|
| 40 | Zr | 锆 | gào | 91.22 | 4d2 5s2 | +4 | 副族 金属 | Zirconium | 氧化物立方氧化锆为钻石的人工替代品 |
|---|
| 41 | Nb | 铌 | ní | 92.91 | 4d4 5s1 | +5 | 副族 金属 | Niobium | 铌钢被用于制作汽车外壳 |
|---|
| 42 | Mo | 钼 | mù | 95.96 | 4d5 5s1 | +4、+6 | 副族 金属 | Molybdenum | 植物生长所需的微量元素 |
|---|
| 43 | Tc | 锝 | dé | 98 | 4d5 5s2 | +4、+7 | 副族 金属 | Technetium | 原子序数最小的放射性元素 |
|---|
| 44 | Ru | 钌 | liǎo | 101.1 | 4d7 5s1 | +1、+4、+8 | Ⅷ族 金属 | Ruthenium | 硬而脆呈浅灰色的多价稀有金属元素 |
|---|
| 45 | Rh | 铑 | lǎo | 102.9 | 4d8 5s1 | +3,+4 | Ⅷ族 金属 | Rhodium | 现代珠宝制作过程进行表面处理的必须元素 |
|---|
| 46 | Pd | 钯 | bǎ | 106.4 | 4d10 | +2、+4 | Ⅷ族 金属 | Palladium | 被应用于酒精检测中 |
|---|
| 47 | Ag | 银 | yín | 107.9 | 4d10 5s1 | +1 | 副族 金属 | Silver | 贵金属,曾经是全球范围内的硬通货,导电性最好 |
|---|
| 48 | Cd | 镉 | gé | 112.4 | 4d10 5s2 | +2 | 副族 金属 | Cadmium | 重金属,过量摄入会导致痛痛病 |
|---|
| 49 | In | 铟 | yīn | 114.8 | 5s2 5p1 | +3 | 主族 金属 | Indium | 可塑性强,有延展性,115In是主要核素,有放射性 |
|---|
| 50 | Sn | 锡 | xī | 118.7 | 5s2 5p2 | +2、+4 | 主族 金属 | Tin | 人类最早发现应用的元素之一,被用于制造容器 |
|---|
| 51 | Sb | 锑 | tī | 121.8 | 5s2 5p3 | -3、+3、+5 | 主族 金属 | Antimony | 熔点低,被用于制作保险丝 |
|---|
| 52 | Te | 碲 | dì | 127.6 | 5s2 5p4 | -2、+4、+6 | 主族 非金属 | Tellurium | 密度最大的非金属 |
|---|
| 53 | I | 碘 | diǎn | 126.9 | 5s2 5p5 | -1、+5、+7 | 主族 非金属 卤素 | Iodine | 活泼,甲状腺所需的微量元素 |
|---|
| 54 | Xe | 氙 | xiān | 131.3 | 5s2 5p6 | +4、+6、+8 | 主族 非金属 稀有气体 | Xenon | 稀有气体 |
|---|
| 55 | Cs | 铯 | sè | 133 | 6s1 | +1 | 主族 金属 碱金属 | Cesium | 活泼 |
|---|
| 56 | Ba | 钡 | bèi | 137.3 | 6s2 | +2 | 主族 金属 碱土金属 | Barium | 硫酸钡被应用于钡餐透视(检查是否胃穿孔) |
|---|
| 57 | La | 镧 | lán | 139 | 5d1 6s2 | +3 | 副族 金属 镧系 | Lanthanum | 第一个镧系元素 |
|---|
| 58 | Ce | 铈 | shì | 140 | 4f1 5d1 6s2 | +3、+4 | 副族 金属 镧系 | Cerium | 用来制造打火石 |
|---|
| 59 | Pr | 镨 | pǔ | 141 | 4f3 6s2 | +3 | 副族 金属 镧系 | Praseodymium | 英文名称最长 |
|---|
| 60 | Nd | 钕 | nǚ | 144 | 4f4 6s2 | +3 | 副族 金属 镧系 | Neodymium | 磁性强 |
|---|
| 61 | Pm | 钷 | pǒ | 145 | 4f5 6s2 | +3 | 副族 金属 镧系 | Promethium | 有放射性 |
|---|
| 62 | Sm | 钐 | shān | 150.5 | 4f6 6s2 | +3 | 副族 金属 镧系 | Samarium | 磁性强 |
|---|
| 63 | Eu | 铕 | yǒu | 152 | 4f7 6s2 | +3 | 副/金/镧 | Europium | 活泼,能放出红光 |
|---|
| 64 | Gd | 钆 | gá | 157 | 4f7 5d1 6s2 | +3 | 副/金/镧 | Gadolinium | 未配对电子达到上限 |
|---|
| 65 | Tb | 铽 | tè | 159 | 4f9 6s2 | +3 | 副/金/镧 | Terbium | 通电时改变形状 |
|---|
| 66 | Dy | 镝 | dī | 162.5 | 4f10 6s2 | +3 | 副/金/镧 | Dysprosium | 英文名称源自“很难得到” |
|---|
| 67 | Ho | 钬 | huǒ | 165 | 4f11 6s2 | +3 | 副/金/镧 | Holmium | 银白色,质软,可用来制磁性材料 |
|---|
| 68 | Er | 铒 | ěr | 167 | 4f12 6s2 | +3 | 副/金/镧 | Erbium | 银灰色,质软,可用来制特种合金,激光器等 |
|---|
| 69 | Tm | 铥 | diū | 169 | 4f13 6s2 | +3 | 副/金/镧 | Thulium | 银白色,质软,可用来制X射线源等 |
|---|
| 70 | Yb | 镱 | yì | 173 | 4f14 6s2 | +2、+3 | 副/金/镧 | Ytterbium | 银白色,质软,可用来制特种合金,也用作激光材料等 |
|---|
| 71 | Lu | 镥 | lǔ | 175 | 4f14 5d1 6s2 | +3 | 副/金/镧 | Lutetium | 银白色,质软,可用于核工业 |
|---|
| 72 | Hf | 铪 | hā | 178.5 | 5d2 6s2 | +4 | 副/金 | Hafnium | 银白色,熔点高。可用来制耐高温合金,也用于核工业等 |
|---|
| 73 | Ta | 钽 | tǎn | 181 | 5d3 6s2 | +5 | 副/金 | Tantalum | 钢灰色,耐腐蚀质硬,熔点高。可用于航天工业及核工业 |
|---|
| 74 | W | 钨 | wū | 184 | 5d4 6s2 | +4、+6 | 副/金 | Tungsten | 稳定元素中熔点最高 |
|---|
| 75 | Re | 铼 | lái | 186 | 5d5 6s2 | +7 | 副/金 | Rhenium | 最晚被发现的稳定元素 |
|---|
| 76 | Os | 锇 | é | 190 | 5d6 6s2 | +4,+6,+8 | 副/金 | Osmium | 密度最大的金属 |
|---|
| 77 | Ir | 铱 | yī | 192 | 5d7 6s2 | +3,+4、+6、+9 | 副/金 | Iridium | 熔点高,质硬而脆。可用来制科学仪器等 |
|---|
| 78 | Pt | 铂 | bó | 195 | 5d9 6s1 | +2,+4 | 副/金 | Platinum | 被应用于珠宝首饰中的贵金属,俗称铂金 |
|---|
| 79 | Au | 金 | jīn | 197 | 5d10 6s1 | +1、+3 | 副/金 | Gold | 化学性质极稳定,人类最早发现及应用的贵金属,全球硬通货 |
|---|
| 80 | Hg | 汞 | gǒng | 200.6 | 5d10 6s2 | +1、+2 | 副/金 | Mercury | 唯一一种在常温下为液态的金属 |
|---|
| 81 | Tl | 铊 | tā | 204.5 | 6s2 6p1 | +3 | 主/金 | Thallium | 银白色,质软。可用来制合金等。铊的化合物有毒 |
|---|
| 82 | Pb | 铅 | qiān | 207 | 6s2 6p2 | +2、+4 | 主/金 | Lead | 密度大,熔点低,对人体有毒性。许多化妆品中必须含有的元素 |
|---|
| 83 | Bi | 铋 | bì | 209 | 6s2 6p3 | +3、+5 | 主/金 | Bismuth | 合金熔点很低,可用来做保险丝和汽锅上的安全塞等 有放射性 |
|---|
| 84 | Po | 钋 | pō | 209 | 6s2 6p4 | -2、+6 | 主/金 | Polonium | 放射 |
|---|
| 85 | At | 砹 | ài | 210 | 6s2 6p5 | +5 | 主/非/卤 | Astatine | 放射、活泼 |
|---|
| 86 | Rn | 氡 | dōng | 222 | 6s2 6p6 | +2 | 主/非/稀 | Radon | 放射 |
|---|
| 87 | Fr | 钫 | fāng | 223 | 7s1 | +1 | 主/金/碱 | Francium | 放射(注:放射性虽短但仍然存在) |
|---|
| 88 | Ra | 镭 | léi | 226 | 7s2 | +2 | 主/金/碱土 | Radium | 放射 |
|---|
| 89 | Ac | 锕 | ā | 227 | 6d1 7s2 | +3 | 副/金/锕 | Actinium | 放射 |
|---|
| 90 | Th | 钍 | tǔ | 232 | 6d2 7s2 | +4 | 副/金/锕 | Thorium | 放射 |
|---|
| 91 | Pa | 镤 | pú | 231 | 5f2 6d1 7s2 | +5 | 副/金/锕 | Protactinium | 放射 |
|---|
| 92 | U | 铀 | yóu | 238 | 5f3 6d1 7s2 | +3、+4,+6 | 副/金/锕 | Uranium | 放射,同位素铀235被用于制作原子弹 |
|---|
| 93 | Np | 镎 | ná | 237 | 5f4 6d1 7s2 | +5、+7 | 副/金/锕 | Neptunium | 放射 |
|---|
| 94 | Pu | 钚 | bù | 244 | 5f6 7s2 | +4、+6、+8 | 副/金/锕 | Plutonium | 放射,用于制作原子弹 |
|---|
| 95 | Am | 镅 | méi | 243 | 5f7 7s2 | +3、+5、+7、+8 | 副/金/锕 | Americium | 人造 放射 用于烟雾报警器中 |
|---|
| 96 | Cm | 锔 | jú | 247 | 5f7 6d1 7s2 | +3、+6、+7 | 副/金/锕 | Curium | 人造 放射 |
|---|
| 97 | Bk | 锫 | péi | 247 | 5f9 7s2 | +3、+5 | 副/金/锕 | Berkelium | 人造 放射 |
|---|
| 98 | Cf | 锎 | kāi | 251 | 5f10 7s2 | +3、+5 | 副/金/锕 | Californium | 人造 放射,最贵金属 |
|---|
| 99 | Es | 锿 | āi | 252 | 5f11 7s2 | +3 | 副/金/锕 | Einsteinium | 人造 放射 |
|---|
| 100 | Fm | 镄 | fèi | 257 | 5s12 7s2 | +3 | 副/金/锕 | Fermium | 人造 放射 |
|---|
| 101 | Md | 钔 | mén | 258 | 5f13 7s2 | +3 | 副/金/锕 | Mendelevium | 人造 放射 |
|---|
| 102 | No | 锘 | nuò | 259 | 5f14 7s2 | +2、+3 | 副/金/锕 | Nobelium | 人造 放射 |
|---|
| 103 | Lr | 铹 | láo | 260 | 5f14 7s15p1 | +3 | 副/金/锕 | Lawrencium | 人造 放射 |
|---|
备注:104~118号元素中部分元素,其汉字简体中文在部分设备上无法查看,故注明其繁体中文或以表意文字描述字符(IDS)描述的简体中文如下附表:
| 原子序数 | 符号 | 简体中文 | 繁体中文或简体IDS | 汉语拼音 | 相对原子质量 | 价电子 | 常见化合价 | 分类 | 英文名 | 简介 |
|---|
| 104 | Rf | | 鑪/鈩 | lú | 261 | 6d2 7s2 | +4 | 副/金 | Rutherfordium | 人造 放射 |
|---|
| 105 | Db | | | dù | 262 | 6d3 7s2 | +5 | 副/金 | Dubnium | 人造 放射 |
|---|
| 106 | Sg | | | xǐ | 263 | 6d4 7s2 | +6 | 副/金 | Seaborgium | 人造 放射 |
|---|
| 107 | Bh | | | bō | 264 | 6d5 7s2 | +7 | 副/金 | Bohrium | 人造 放射 |
|---|
| 108 | Hs | | | hēi | 265 | 6d6 7s2 | +8 | 副/金 | Hassium | 人造 放射 |
|---|
| 109 | Mt | 鿏 | 䥑 | mài | 266 | 6d7 7s2 | 0 | 副/金 | Meitnerium | 人造 放射 |
|---|
| 110 | Ds | | 鐽 | dá | 269 | 6d8 7s2 | 0 | 副/金 | Darmstadtium | 人造 放射 |
|---|
| 111 | Rg | | 錀 | lún | 272 | 6d9 7s2 | 0 | 副/金 | Roentgenium | 超重元素 |
|---|
| 112 | Cn | 鿔 | 鎶 | gē | 277 | 6d10 7s2 | 0 | 副/金 | Copernicium | 超重元素 |
|---|
| 113 | Nh | 鿭[10] | 鉨[14] | nǐ | 286[15] | 5f146d107s27p1[15] | +3,+1 | 主/金 | Nihonium[14] | 不稳定的超重元素,人造 放射 |
|---|
| 114 | Fl | | 鈇[16] | fū | 289[16] | 5f146d107s27p2[16] | ,0,+3 | 主/金 | Flerovium[16] | 第一种表现出惰性气体特征的超重元素,人造 放射 |
|---|
| 115 | Mc | 镆[14] | ⿰钅莫 | mò | 289[17] | 5f146d107s27p3[17] | +1,+3 | 主/金 | Moscovium[14] | 人工合成的放射性金属元素,人造 放射 |
|---|
| 116 | Lv | [11] | 鉝[18] | lì[18] | 293[19] | 5f146d107s27p4[19] | +4 | 主/金 | Livermorium[19] | 人工合成的放射性化学元素,人造 放射 |
|---|
| 117 | Ts | 鿬[12] | ⿰石田[14] | tián | 294[20] | 5f146d107s27p5[20] | -1 | 主/非/卤 | Tennessine[14] | 卤族元素,人造 放射 |
|---|
| 118 | Og | 鿫[13] | ⿹气奥[14] | ào | 294[21] | 5f146d107s27p6[21] |
| 主/非/稀 | Oganesson[14] | 人工合成的稀有气体元素,人造 放射 |
|---|
Luminescence Is Cold Light

Cold light is visible light produced by processes
other than heating. Light sticks are an example of cold light, which is
produced when chemicals are mixed together and the energy released is in
the form of visible light.
The firefly in the photo gives off light that like the light stick is
produced by a chemical reaction with visible light being the released
energy.
Luminescence refers to any cold light. The term ‘luminescence’ was introduced in 1888 by Eilhard Wiedemann.
Luminescence can be caused by: chemical reactions, electrical energy, subatomic motions, or stress on a crystal.
Types of Luminescence
Bioluminescence, light emission by a living organism, such as fireflies.

Chemiluminescence, a result of a chemical reaction,
such as glow sticks or luminol plus hemoglobin (chemical in blood). The
glowing containers shown here contain mixtures of blood and luminol.
Electroluminescence: Light produced when an electric
current is passed through a substance. An electroluminescent (EL)
device is similar to a laser in that photons(units of light energy)
are produced by the return of an excited electron to its ground state,
but unlike lasers EL devices need much less energy to operate and do not
produce coherent light. One kind of EL is an LED (light emitting
diode), such as Christmas tree lights. LEDs present many advantages over incandescent light sources including lower energy consumption and longer lifetime.
Cathodoluminescence is an optical and electrical
phenomenon whereby a beam of electrons is generated by an electron gun
(e.g., cathode ray tube) and then impacts on a luminescent material such as a phosphor, causing the material to emit visible light.
Mechanoluminescence is visible light emission resulting from any mechanical action on a solid. Examples are:
Triboluminescence is an optical (visual) phenomenon in which visual light is generated when chemical
bonds in a material are broken as a result of the material being pulled
apart, ripped, scratched, crushed, or rubbed. Scientists do not fully
understand this light producing process, but it is believed that the
light results from the separation of electric charges leaving one side
of the fracture positively charged and the other negatively charged. If
the charge build up is great enough, an electric discharge across the
gap occurs with the emission of light energy.
Triboluminescence can be observed when breaking sugar crystals (especially Wint-O-Green Life Savers) and peeling adhesive tapes.
Fractoluminescence and triboluminescence produce
light in the same way. The difference is that fractoluminescence refers
only to cold light produced by the fracturing of materials.
Piezoluminescence is caused by pressure that results only in elastic deformation.
Elastic deformation means the material can be stretched or twisted out
of shape, but when the deforming pressure is removed, the material
returns to its original shape. Thus, piezoluminescence differs from
triboluminescence in that the material does not break.
Photoluminescence, a result of absorption of photons (units of radiant energy) by substances that are fluorescent or phosphorescent.
Fluorescence,
is the emission of photons (units of radiant energy) that are
perceived as colors of light. This occurs as a result of high energy
photons, such as Ultraviolet radiation (UV light) striking phosphors,
which are chemicals that absorb high energy photons and emit lower energy photons (visible light).Note: Materials that fluoresce only do so during the time that the high energy radiant energy is being absorbed.
Examples of Fluorescence
1. The exoskeleton of scorpions contain phosphors. At night, scorpions can be located using a black light. The UV radiation emitted by this light causes the scorpion to fluoresce.
2. See, Glo Germs for ideas for teaching kids proper hand washing as well as how germs can spread.
3. A Fluorescent Powder is Used to Represent Germs. This powder as well as a black light can be purchased from Educational Innovation online.
Phosphorescence, like fluorescence, is visible light emitted as excited electrons return to their normal positions called ground state. The difference being that the release of energy is slower for phosphorescence. While materials that fluoresce stop emitting light as soon as the exciting energy is removed, phosphorescent
materials continue to glow for seconds to hours after the excitation
energy is removed. Thus, these materials will glow-in-the-dark.
Phosphorescent Pigment can be purchased from
“Education Innovation” online. You will find information about phosphorescence and ideas for different experiments.
Fluorescence, phosphorescence, and photoluminescence occur when a
sample is excited by absorbing photons and then emits them with a decay
time that is characteristic of the sample environment. Fluorescence is a
term used by chemists when the absorbing and emitting species is an
atom or molecule. Phosphorescence is similar to fluorescence, except
that the time between absorption and emission is much longer than in
fluorescence. Photoluminescence is the term physicists use to describe
the absorption and emission of light by things such as semiconductors
and nanotubes. Regardless of the terminology, when samples absorb
photons and then emit them at a different wavelength the resultant light
can be dispersed by a spectrograph, the spectrum can be detected by a
device such as a CCD, and information can be gleaned about the sample.
As illustrated in the diagram at right, fluorescence occurs when a
chemical species absorbs a photon and is excited to a singlet electronic
excited state, relaxes via non-radiative mechanisms, emits a
lower-energy photon, and then transitions to the ground electronic
state. In fluorescence, the time between absorption and emission is on
the order of nanoseconds. The spectrum of the wavelengths emitted can be
used to identify atoms and molecules, as well as to determine chemical
structures. The intensity of the photons emitted can be used to
determine the concentration of chemical species.


Since absorption is a requirement for the fluorescence process,
molecules and functional groups that are strong UV-Vis absorbers can be
strong fluorescers. For example, molecules with extended pi-electron
systems, including aromatic and conjugated aromatic rings, make
excellent fluorescers (known as fluorophores). In biology, fluorophores
are attached to molecules such as proteins, are excited, and the
resultant fluorescence used to image molecules and cells. Spectral
analysis of this fluorescence can give chemical information about
biological systems.
Phosphorescence
Phosphorescence is similar to fluorescence except the time between
photon absorption and emission is from seconds to hours rather than
nanoseconds. Like fluorescence, phosphorescence begins with absorption
by a photon and excitation to a singlet electronic state. Via a process
called intersystem crossing, the singlet state couples to a triplet
electronic excited state, which then gives off a photon and relaxes to
the ground electronic state. Singlet-triplet coupling is “forbidden”
(which is responsible for the time delay in phosphorescence compared to
fluorescence). The amino acid tryptophan phosphoresces, so
phosphorescence can be used to study proteins.
Photoluminescence
Photoluminescence is fluorescence as applied to semiconductors and
other materials. Dispersing the photoluminescent light to form a
spectrum can give information such as the purity of semiconductors and
the structure of nanotubes.